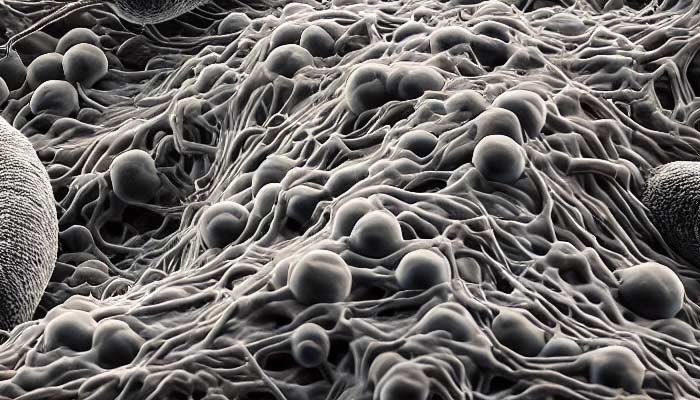
Lyme disease and other borreliosis-related illnesses have become significant public health concerns worldwide. The genus Borrelia includes a diverse group of spirochete bacteria, such as Borrelia burgdorferi, Borrelia afzelii, Borrelia garinii, and Borrelia miyamotoi, among others. These pathogens are responsible for various tick-borne diseases and exhibit remarkable adaptability, enabling them to persist in different host environments and evade immune responses. A crucial aspect of their survival strategy is the formation of biofilms—a structured community of bacterial cells encased within a self-produced extracellular matrix.
Dissolving Borrelia Biofilms with Enzymes
Understanding the mechanisms of biofilm formation by Borrelia species is essential for developing effective diagnostic and therapeutic strategies. Biofilms contribute to the persistence of infections, antibiotic resistance, and immune evasion, posing significant challenges to treatment. This chapter explores the formation, characteristics, and clinical implications of Borrelia biofilms, integrating general concepts of bacterial biofilm formation with specific insights into Borrelia species. Recent studies have provided compelling evidence of Borrelia biofilms in real-world settings, highlighting their role in the pathogenesis of Lyme disease and other borrelioses.
Biofilm Formation in Bacteria
Biofilms are complex, multicellular communities of microorganisms that adhere to surfaces and are embedded within a self-produced matrix of extracellular polymeric substances (EPS). This mode of growth is distinct from the planktonic, or free-living, state and confers several advantages to the microbial community, including enhanced survival under adverse conditions.
The formation of a biofilm is a dynamic and multifaceted process involving several stages:
-
Initial Attachment: Planktonic bacteria encounter a surface and adhere to it through weak, reversible interactions such as van der Waals forces and electrostatic attractions. Surface properties, including roughness and hydrophobicity, influence the likelihood and strength of initial attachment. Environmental factors like nutrient availability and fluid dynamics also play critical roles.
-
Irreversible Adhesion: Bacteria strengthen their attachment by producing cell surface structures like pili, fimbriae, and adhesins, facilitating strong, specific interactions with the surface. Concurrently, they begin synthesizing EPS, anchoring themselves more firmly and initiating the formation of the biofilm matrix.
-
Biofilm Maturation: The bacterial cells proliferate and continue to produce EPS, leading to the formation of microcolonies and a complex three-dimensional structure. The biofilm architecture includes channels that allow for nutrient distribution and waste removal. Within this environment, bacteria exhibit phenotypic diversity, differentiating into subpopulations with distinct metabolic activities.
-
Dispersion: The final stage involves the dispersal of bacteria from the mature biofilm back into the planktonic phase. Dispersion can occur through passive mechanisms, such as erosion due to shear forces, or active processes involving the expression of enzymes that degrade the EPS matrix. This release allows bacteria to colonize new sites, promoting the spread of biofilm-associated infections.
The EPS matrix is a heterogeneous mixture primarily composed of polysaccharides, proteins, extracellular DNA (eDNA), and lipids. This matrix provides structural support, mediates interactions within the microbial community, and offers protection against environmental stressors, antimicrobial agents, and the host immune system.
Regulation of Biofilm Formation
Biofilm development is governed by complex genetic regulatory networks that respond to environmental cues. Key regulatory mechanisms include quorum sensing, two-component regulatory systems, and secondary messenger molecules like cyclic di-GMP. These systems enable bacteria to coordinate communal behaviors, adapt to changing conditions, and regulate the expression of genes involved in adhesion, EPS production, and stress responses.
Biofilms in Pathogenesis and Antimicrobial Resistance
Biofilms play a critical role in the pathogenesis of many bacterial infections. They contribute to chronic infections by enabling bacteria to persist on tissues and medical devices, leading to long-term infections that are difficult to eradicate. The biofilm matrix shields bacteria from immune detection and phagocytosis, allowing them to evade the host immune system. Additionally, biofilm-associated bacteria exhibit heightened resistance to antimicrobial agents due to factors such as the physical barrier of the EPS matrix, metabolic heterogeneity, and the expression of efflux pumps and antibiotic-degrading enzymes.
Borrelia Biofilms: Evidence and Characteristics
Recent studies have provided substantial evidence that Borrelia species can form biofilms both in vitro and in vivo. This capability has significant implications for the persistence and treatment resistance of Lyme disease and other borrelioses.
In Vitro Observations
Laboratory studies have shown that Borrelia species can form biofilm-like aggregates under specific conditions. When cultured in vitro, these bacteria exhibit aggregation and produce EPS, key features of biofilm formation. Advanced imaging techniques, such as confocal laser scanning microscopy and scanning electron microscopy, have visualized three-dimensional biofilm structures. Staining methods targeting polysaccharides and eDNA confirm the presence of EPS components within these aggregates. Environmental stressors, including nutrient limitation, pH changes, and exposure to sub-inhibitory concentrations of antibiotics, can induce Borrelia to form complex biofilm-like structures, suggesting that biofilm formation is a stress response mechanism aiding bacterial survival.
In Vivo Evidence
Emerging evidence indicates that Borrelia biofilms are present in the infected tissues of patients with Lyme disease. A study published in 2019 demonstrated the presence of Borrelia biofilms in human skin biopsies from patients with erythema migrans, the characteristic rash of early Lyme disease. Using advanced imaging and specific staining, researchers confirmed that Borrelia can form biofilms in vivo. Biofilm-like structures have also been observed in the cardiac and neurological tissues of infected animal models, supporting the hypothesis that Borrelia biofilms contribute to the dissemination and persistence of infection within the host.
Molecular Evidence
Molecular studies have identified genes and proteins potentially involved in Borrelia biofilm formation. Transcriptomic analyses reveal that genes associated with EPS biosynthesis, adhesion, and stress responses are upregulated during biofilm development. Proteomic studies have detected proteins related to biofilm structural components and regulatory pathways. The identification of cyclic di-GMP signaling pathways in Borrelia suggests that these bacteria utilize similar regulatory mechanisms as other biofilm-forming species to control biofilm development.
Structural Characteristics of Borrelia Biofilms
In vitro, Borrelia biofilms exhibit a heterogeneous architecture with microcolonies embedded in an EPS matrix. The structure includes water channels facilitating nutrient distribution and waste removal. Advanced imaging has revealed that Borrelia biofilms form complex, protective niches composed of densely packed spirochetes surrounded by an extracellular matrix rich in polysaccharides, proteins, and nucleic acids. This architecture allows the establishment of microenvironments that protect bacteria from external threats, including immune responses and antimicrobial agents.
The EPS matrix of Borrelia biofilms comprises various biomolecules contributing to stability and function:
- Polysaccharides provide structural integrity and mediate adhesion. Alginate-like substances identified in Borrelia biofilms confer resistance to environmental stresses.
- Proteins, including adhesins and enzymes, may modify the matrix or aid in nutrient acquisition. Outer surface proteins (Osps) involved in adhesion to host tissues may play roles in biofilm formation.
- Extracellular DNA (eDNA) contributes to matrix stability and facilitates horizontal gene transfer. Detection of eDNA within Borrelia biofilms indicates its importance in biofilm integrity.
- Lipids and Vesicles influence hydrophobic interactions and carry signaling molecules. Membrane vesicles containing lipoproteins may participate in intercellular communication and immune modulation.
Within Borrelia biofilms, cells display phenotypic diversity. Metabolically active cells are typically located near the biofilm surface, engaged in growth and EPS production. Dormant persister cells reside deeper within the biofilm, exhibiting reduced metabolic activity and increased tolerance to antibiotics. This heterogeneity contributes to the biofilm's resilience and complicates treatment efforts, as dormant cells can evade antimicrobial agents targeting active processes.
Clinical Implications of Borrelia Biofilms
Chronic Infection and Persistence
Biofilm formation plays a critical role in the chronicity of Borrelia infections. The protective environment of the biofilm shields bacteria from antibiotics and the immune system, allowing persistence despite treatment. Patients with prolonged symptoms after standard antibiotic therapy, sometimes referred to as Post-Treatment Lyme Disease Syndrome (PTLDS), may be experiencing the effects of biofilm-protected bacteria. The presence of Borrelia biofilms in various tissues suggests they may serve as reservoirs for ongoing infection, facilitating dissemination to different organs and contributing to the diverse clinical manifestations of Lyme disease.
Antibiotic Resistance
Biofilms contribute to antibiotic resistance through multiple mechanisms. The EPS matrix acts as a physical barrier, impeding antibiotic penetration. Nutrient and oxygen gradients within the biofilm create zones of slow-growing or dormant cells less susceptible to antibiotics. Biofilm-associated cells may express higher levels of efflux pumps and antibiotic-degrading enzymes. Studies have demonstrated that Borrelia biofilms exhibit significantly higher antibiotic tolerance compared to planktonic cells, underscoring the need to consider biofilm formation when designing treatment regimens.
Immune Evasion
The biofilm matrix can mask bacterial antigens, reducing recognition by the immune system. Borrelia biofilms may inhibit phagocytosis by immune cells and alter cytokine production, contributing to immune evasion and persistent infection. Biofilms can modulate immune responses, potentially leading to chronic inflammation and tissue damage.
Diagnostic Challenges
Biofilm-associated Borrelia may be difficult to detect using standard diagnostic methods. The low bacterial load released from biofilms can result in false-negative results in culture and molecular assays. Altered antigen expression may affect serological test sensitivity. Biofilm localization in tissues complicates sampling, as invasive procedures may be required to obtain specimens containing biofilm communities. Improved diagnostic techniques, such as advanced imaging and molecular methods targeting biofilm-specific markers, are needed to accurately identify biofilm-associated infections.
Enzymatic Strategies for Disrupting Borrelia Biofilms
Introduction
The persistence and resilience of Borrelia biofilms present significant challenges in the treatment of Lyme disease and other borrelioses. Traditional antibiotic therapies often fail to eradicate these biofilms, leading to chronic infections and treatment failures. Enzymatic disruption of biofilms has been proposed as a promising strategy to overcome these challenges by targeting the structural components of the biofilm matrix. By degrading the extracellular polymeric substances (EPS) that confer protection to the bacterial community, enzymes could potentially enhance the efficacy of antimicrobial agents and facilitate immune clearance.
While enzymatic treatments have shown effectiveness against biofilms formed by other bacterial species, the application of this approach to Borrelia biofilms remains largely theoretical. Limited research has been conducted to evaluate the efficacy of specific enzymes in disrupting Borrelia biofilms, and the existing studies are preliminary. This chapter explores the theoretical basis for using enzymes to dissolve Borrelia biofilms, discusses the potential enzymes involved, and highlights the need for further research to validate this approach.
Theoretical Basis for Enzymatic Disruption
Biofilms are composed of a complex mixture of extracellular polymeric substances, including polysaccharides, proteins, extracellular DNA (eDNA), and lipids. Enzymes capable of degrading these components have been effective in disrupting biofilms of various bacterial species, such as Staphylococcus aureus and Pseudomonas aeruginosa. The success of enzymatic treatments in these contexts suggests a potential application for Borrelia biofilms.
Potential Enzymes for Targeting Borrelia Biofilms
Polysaccharide-Degrading Enzymes
Polysaccharides are a major component of the biofilm matrix, providing structural support and mediating cell adhesion. Enzymes such as alginate lyase and dispersin B have been shown to degrade polysaccharides in biofilms of other bacteria. For instance, alginate lyase targets alginate, a key polysaccharide in Pseudomonas biofilms. Dispersin B hydrolyzes poly-N-acetylglucosamine, involved in Staphylococcus biofilms.
In the context of Borrelia, the specific polysaccharides present in the biofilm matrix are not fully characterized. Therefore, the identification of suitable polysaccharide-degrading enzymes for Borrelia biofilms remains speculative. Further research is needed to elucidate the polysaccharide composition of Borrelia biofilms to select appropriate enzymatic targets.
Proteases
Proteins within the biofilm matrix serve structural roles and facilitate adhesion. Proteases, such as trypsin and proteinase K, have been used experimentally to disrupt biofilms by degrading matrix proteins. In other bacterial species, protease treatment has reduced biofilm biomass and increased susceptibility to antibiotics.
For Borrelia biofilms, the effectiveness of proteases is theoretical at this stage. Studies on the protein composition of the Borrelia biofilm matrix are limited, and the impact of protease treatment on Borrelia biofilms has not been extensively investigated.
DNases
Extracellular DNA contributes to the structural integrity of biofilms and facilitates genetic exchange. DNase I, an enzyme that degrades DNA, has shown effectiveness in disrupting biofilms of various bacteria. In experimental settings, DNase I treatment has reduced biofilm biomass and enhanced antibiotic penetration.
Regarding Borrelia biofilms, the role of eDNA is not well-defined. While eDNA is likely a component of the biofilm matrix, as in other bacteria, there is a lack of specific studies demonstrating the effectiveness of DNase I against Borrelia biofilms. The potential of DNase treatment remains a theoretical consideration that requires empirical validation.
Lipases
Lipids and membrane vesicles in the biofilm matrix may contribute to biofilm stability and facilitate communication. Lipases, which hydrolyze lipids, could theoretically disrupt these components. However, there is minimal research on the use of lipases for biofilm disruption, particularly concerning Borrelia biofilms.
Theoretical Considerations
The application of enzymatic treatments to Borrelia biofilms is based on the following theoretical considerations:
-
Structural Similarities: Biofilms of different bacteria share common structural components, such as polysaccharides and eDNA. Enzymes effective against these components in other biofilms might have similar effects on Borrelia biofilms.
-
Mechanistic Plausibility: Enzymes can degrade biofilm matrix components, potentially weakening the biofilm and exposing bacteria to antibiotics and immune responses.
-
Synergistic Potential: Combining enzymatic treatments with antibiotics could enhance bacterial eradication, as observed in studies with other bacterial biofilms.
Challenges and Limitations
Several challenges must be addressed to advance the application of enzymatic treatments for Borrelia biofilms:
-
Lack of Specificity: Without detailed knowledge of the biofilm matrix composition, selecting effective enzymes is difficult.
-
Safety Concerns: Enzymes must be safe for human use, without causing damage to host tissues or eliciting adverse immune responses.
-
Delivery Methods: Effective delivery systems are needed to ensure that enzymes reach the biofilm site in active form.
-
Resistance Development: The potential for bacteria to develop resistance to enzymatic treatments requires consideration.
Future Directions
To move from theoretical concepts to practical applications, the following steps are necessary:
-
Characterization of Borrelia Biofilm Matrix: Detailed studies to identify the specific components of the Borrelia biofilm matrix will inform the selection of appropriate enzymes.
-
In Vitro Testing: Controlled laboratory experiments to assess the efficacy of selected enzymes against Borrelia biofilms.
-
In Vivo Studies: Animal models to evaluate the safety and effectiveness of enzymatic treatments in a physiological context.
-
Clinical Trials: Rigorous testing in human subjects to determine therapeutic potential, dosing, and safety profiles.
-
Development of Delivery Systems: Innovation in drug delivery technologies to enhance enzyme stability and targeting.
Conclusion
The use of enzymes to disrupt Borrelia biofilms remains a theoretical strategy with potential benefits. While enzymatic treatments have shown promise against biofilms of other bacterial species, their application to Borrelia biofilms requires extensive research. Current evidence is insufficient to confirm the efficacy of enzymes in disrupting Borrelia biofilms. Therefore, these concepts should be regarded as speculative until validated by empirical studies.
Advancing this field will depend on a concerted effort to understand the unique properties of Borrelia biofilms, identify suitable enzymatic targets, and develop safe and effective treatment protocols. The integration of enzymatic approaches into the management of Lyme disease holds the promise of improving outcomes for patients with biofilm-associated infections.