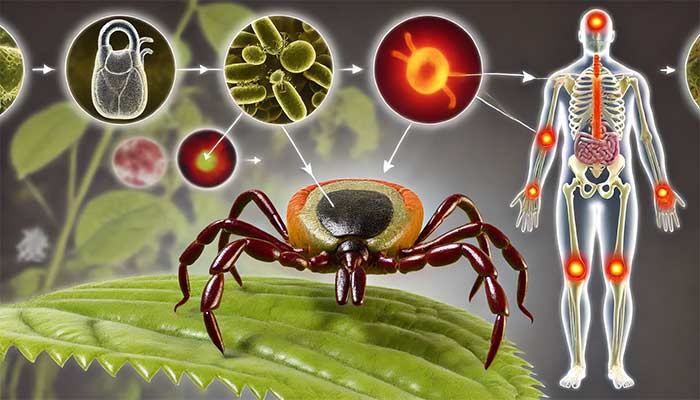
Lyme disease co-infections, such as Babesia and Bartonella, significantly complicate diagnosis and treatment. Various Babesia species, including Babesia microti, Babesia duncani, and others, along with Bartonella strains, worsen Lyme disease symptoms like fatigue, anemia, and neurological issues. These co-infections make the disease harder to diagnose and treat effectively. This chapter explores multiple tick-borne pathogens, including Anaplasma, Ehrlichia, and Rickettsia, and their impact on the immune system, helping healthcare providers develop better diagnostic and treatment strategies for chronic Lyme disease patients.
Tick-Borne Co-Infections in Lyme Disease: Babesia, Bartonella, Treatment & Symptoms
Tick-borne diseases, particularly Lyme disease caused by Borrelia species, rarely occur in isolation. Multiple pathogens are frequently transmitted by the same vectors—particularly Ixodes ticks—leading to co-infections that alter the course of disease, complicate diagnosis, and challenge treatment. Co-infections can intensify symptom severity, delay recovery, and even mask the presence of one pathogen due to overlapping clinical presentations. Lyme patients, often suffering from chronic symptoms, must navigate a multifaceted web of pathogens.
Babesia: A Diverse Genus of Protozoan Parasites
Species Diversity in Babesia
While Babesia microti is the most recognized species in human babesiosis, particularly in North America, Babesia infections are caused by a spectrum of species globally. Recent studies have highlighted that various Babesia species are geographically distributed and exhibit differing levels of pathogenicity. This is crucial for understanding the full spectrum of babesiosis in Lyme patients.
In the U.S., in addition to B. microti, Babesia duncani has been increasingly recognized, particularly in the western United States. Clinical cases of B. duncani appear to be more severe compared to B. microti, often leading to higher rates of complications. Babesia divergens is a prominent cause of babesiosis in Europe and is known to cause more aggressive illness, especially in splenectomized patients. Other species, such as Babesia venatorum and Babesia odocoilei, have also been documented in human cases, but their prevalence is less understood due to limited surveillance and underdiagnosis.
An emerging area of research involves zoonotic transmission and wildlife reservoirs. Babesia species are frequently found in wildlife (such as deer and small mammals), which serve as reservoirs for these parasites. This complicates disease management, as the range of potential Babesia strains capable of infecting humans is likely much broader than currently recognized. The increasing awareness of genetic diversity within Babesia has spurred research into the pathogen's adaptability, transmission, and host-parasite interactions.
Pathophysiology and Immune Evasion of Babesia
Babesia is an intraerythrocytic parasite, meaning it infects red blood cells (RBCs). Once inside the RBCs, Babesia undergoes a cycle of replication, leading to the destruction of the host cell. The resulting hemolysis (destruction of RBCs) contributes to many of the clinical symptoms, such as anemia, jaundice, and fatigue.
One of the challenges in treating Babesia is the pathogen's ability to evade the host immune system. Babesia utilizes antigenic variation, whereby it alters its surface proteins to avoid detection by the immune system. This immune evasion allows for prolonged infections, even after apparent clinical resolution. In addition, Babesia can persist at low levels in the blood for months or even years, contributing to relapses. Chronic infection is particularly problematic in immunocompromised patients and those without a spleen, as the spleen plays a critical role in clearing parasitized RBCs.
Emerging research has begun to explore the interaction between Babesia and the human immune system in greater detail. Studies show that Babesia suppresses the innate immune response, which includes the activation of macrophages and natural killer (NK) cells, both of which are essential for controlling parasitic infections. This immune suppression may also explain the high rate of co-infections with other pathogens, as a weakened immune system can struggle to eliminate multiple invaders simultaneously.
Bartonella: An Expanding Genus of Pathogens
Species Diversity in Bartonella
Bartonella is an often under-recognized but significant co-infection in Lyme disease. Historically, Bartonella henselae—the agent of cat scratch disease—was considered the most relevant to human disease. However, recent research has unveiled a much wider spectrum of Bartonella species capable of infecting humans, each associated with distinct clinical manifestations. Bartonella is now known to cause chronic, systemic infections with a broad array of symptoms.
- Bartonella henselae: The most well-known species, responsible for cat scratch disease. It typically causes lymphadenopathy and flu-like symptoms but can also lead to neurological and musculoskeletal complications.
- Bartonella quintana: Transmitted by lice and associated with trench fever, B. quintana is increasingly recognized as a cause of endocarditis and chronic infections in homeless populations. It can also be transmitted by ticks and may play a larger role in Lyme co-infections than previously recognized.
- Bartonella bacilliformis: Causes Carrion’s disease, a life-threatening illness endemic to parts of South America, transmitted by sandflies. While it has limited geographical distribution, its ability to cause systemic vascular infection is notable.
- Bartonella vinsonii, Bartonella elizabethae, Bartonella koehlerae, and others: These species have been linked to chronic infections involving the cardiovascular, neurological, and musculoskeletal systems.
Pathogenesis and Immune Response
Bartonella species are gram-negative bacteria with a unique ability to invade endothelial cells (the cells lining blood vessels) and erythrocytes, causing vascular inflammation and persistent bacteremia. The bacterium’s ability to hide within cells allows it to evade immune detection and persist in the host for long periods, often leading to chronic infection.
One of the key challenges in understanding Bartonella is its ability to form intraerythrocytic colonies, much like Babesia, allowing it to evade the immune system and persist intracellularly. Bartonella’s unique virulence factors include proteins that disrupt the endothelial cell structure and facilitate the formation of vascular lesions. This has led researchers to explore the potential role of Bartonella in vasculitis and other inflammatory conditions that are poorly understood in the context of Lyme disease.
Emerging research suggests that Bartonella co-infections may exacerbate Lyme-related symptoms by intensifying inflammatory responses, particularly in the vascular and neurological systems. This is especially relevant given that chronic Lyme disease often involves neurological symptoms such as brain fog, cognitive dysfunction, and mood disturbances, which can be worsened by the neurotropic nature of Bartonella.
Challenges in Diagnosing Bartonella
Diagnosing Bartonella infections remains a significant challenge. Many diagnostic tests rely on serological assays that may miss chronic or low-level infections. Standard blood cultures are often insufficient because Bartonella species are slow-growing and difficult to detect in typical laboratory settings. PCR testing is more sensitive, but even PCR can produce false negatives due to the bacterium's intracellular hiding mechanisms. Additionally, co-infections with other pathogens, such as Borrelia or Babesia, can mask Bartonella symptoms, leading to underdiagnosis.
Rickettsia: Expanding Beyond Rocky Mountain Spotted Fever
Rickettsia is another important genus of tick-borne pathogens that is underappreciated in the context of Lyme disease co-infections. While Rickettsia rickettsii, the causative agent of Rocky Mountain spotted fever (RMSF), is the most well-known, several other Rickettsia species have been identified as potential co-infections in Lyme patients.
Species and Emerging Recognition
- Rickettsia rickettsii: Known for causing RMSF, it can lead to severe vasculitis, multi-organ failure, and even death if not treated promptly. RMSF is often characterized by a rash, fever, and systemic symptoms.
- Rickettsia parkeri: Transmitted by Amblyomma ticks, it causes a milder form of spotted fever, often mistaken for Lyme disease due to its overlapping symptoms of fever, rash, and malaise.
- Rickettsia felis: Carried by fleas, this pathogen causes flea-borne spotted fever, an emerging disease that has been reported globally.
Recent studies have found evidence of multiple Rickettsia species in ticks, expanding the known range of tick-borne rickettsioses. Rickettsia infections are known to affect the vascular endothelium, leading to symptoms like fever, headache, rash, and vasculitis. However, Rickettsia co-infections with Borrelia or Babesia may complicate clinical presentation, as the overlapping symptoms may lead to misdiagnosis or delayed treatment.
Diagnosis typically involves serology or PCR, though cross-reactivity with other spotted fever group rickettsioses can lead to false positives. Doxycycline remains the treatment of choice for rickettsial infections, but recognizing these co-infections early is critical for avoiding severe complications.
Mycoplasma: An Intracellular Parasite with Systemic Consequences
Mycoplasma species, particularly Mycoplasma pneumoniae and Mycoplasma fermentans, are increasingly implicated in Lyme co-infections. Mycoplasma are among the smallest bacteria capable of independent replication and lack a cell wall, making them highly adaptable to various tissues in the human body.
Clinical Presentation and Co-Infection Complexity
Mycoplasma infections are often associated with respiratory symptoms, but they can also cause systemic symptoms such as joint pain, chronic fatigue, and neurological issues. Lyme patients co-infected with Mycoplasma often experience exacerbated fatigue, cognitive difficulties, and musculoskeletal pain. This makes Mycoplasma a critical pathogen to consider when patients with Lyme disease present with unexplained systemic symptoms, particularly when they do not respond fully to standard Lyme treatments.
Emerging research suggests that Mycoplasma co-infections can persist due to the bacteria’s ability to form biofilms and hide within host cells, similar to Borrelia. Co-infection with Mycoplasma can also trigger immune dysregulation, further complicating the host's response to multiple pathogens.
Diagnosis is often made through PCR testing, as serological tests can be unreliable due to the bacterium's immune evasion tactics. Treatment for Mycoplasma typically involves antibiotics such as doxycycline, azithromycin, or fluoroquinolones, though biofilm disruption strategies may be needed in chronic cases.
Chlamydia: A Significant but Overlooked Co-Infection
Chlamydia species, particularly Chlamydia pneumoniae, have emerged as important co-infections in patients with Lyme disease. C. pneumoniae is an intracellular bacterium associated with respiratory infections but has also been implicated in chronic inflammatory diseases, including atherosclerosis and chronic fatigue syndrome.
Recent studies have explored the role of Chlamydia as a co-infection in tick-borne diseases. Like Mycoplasma, Chlamydia species are intracellular pathogens that can evade the immune system and persist in host tissues. Co-infections with Chlamydia pneumoniae may exacerbate the chronic inflammation seen in Lyme disease, contributing to symptoms such as persistent fatigue, brain fog, and muscle pain.
Diagnosis of Chlamydia infections often involves PCR testing, as serological assays may not distinguish between past and current infections. Treatment usually consists of antibiotics like doxycycline or azithromycin, though the intracellular nature of the bacterium means that prolonged treatment may be necessary.
Other Notable Co-Infections: Anaplasma and Ehrlichia
Anaplasma phagocytophilum and Ehrlichia chaffeensis are intracellular bacteria that infect white blood cells, causing human granulocytic anaplasmosis (HGA) and human monocytic ehrlichiosis (HME), respectively. These infections often occur concurrently with Lyme disease, complicating the clinical presentation.
Anaplasma and Ehrlichia Species
- Anaplasma phagocytophilum: Causes HGA, which is characterized by fever, chills, muscle pain, and leukopenia. HGA can become severe, particularly in older adults or immunocompromised individuals.
- Ehrlichia chaffeensis: Causes HME, a similar disease that also presents with fever, headache, and malaise. Severe cases can result in organ damage or death if not treated promptly.
Both infections are typically diagnosed through PCR testing or serology. Doxycycline is the treatment of choice for both Anaplasma and Ehrlichia infections, and early treatment is crucial for preventing severe outcomes.
Conclusion: Navigating the Complex Landscape of Lyme Co-Infections
Co-infections in Lyme disease patients are not merely an academic concern but a clinical reality that complicates diagnosis and treatment. The interplay between Babesia, Bartonella, Rickettsia, Mycoplasma, Chlamydia, and other pathogens significantly alters the disease course and contributes to the persistence of symptoms. An accurate diagnosis requires a high index of suspicion and comprehensive testing for all relevant pathogens.
Future research must continue to explore the complex interactions between these co-infections and their effects on human immune function, treatment resistance, and chronic symptomatology. By addressing the full spectrum of co-infections, clinicians can move toward more personalized and effective treatment protocols that account for the unique challenges posed by these pathogens.
Modern Treatment Strategies for Tick-Borne Co-Infections
Introduction
Treating tick-borne co-infections presents unique challenges, given the diversity of pathogens and their ability to persist, evade the immune system, and resist standard treatments. Recent research has identified new strategies and therapies that show promise in tackling these infections, especially in chronic cases where traditional antibiotics fall short. In this chapter, we will explore cutting-edge treatments for Babesia, Bartonella, Rickettsia, Mycoplasma, Chlamydia, Anaplasma, and Ehrlichia. Particular attention will be given to the latest findings on biofilm disruption, the use of novel drug combinations, and immune modulation.
Babesia Treatment: New Antiparasitic Strategies
Traditionally, Babesia infections have been treated with combinations of atovaquone and azithromycin for mild to moderate cases, and clindamycin with quinine for severe infections. However, treatment failures and relapses remain common due to Babesia's ability to persist within red blood cells.
Emerging Therapies for Babesia
- Tafenoquine: Originally developed for malaria, tafenoquine has recently shown promise in treating Babesia infections, especially in combination with atovaquone. It targets the persistent forms of Babesia and may help reduce relapse rates.
- Ivermectin: Known for its antiparasitic properties, ivermectin has gained attention for its potential efficacy against Babesia. While still under investigation, ivermectin may prove useful in combination therapy for drug-resistant Babesia strains.
- Methylene Blue: Methylene blue, which has demonstrated efficacy against both malaria and protozoal infections, is now being explored for treating Babesia. It works by disrupting the parasite's metabolism and has shown promising results in clearing persistent Babesia infections in vitro.
Bartonella Treatment: Targeting Persistence and Biofilms
Bartonella infections, especially chronic cases, are notoriously difficult to treat due to the bacteria's ability to form biofilms and enter a stationary phase where they evade standard antibiotics like rifampin and doxycycline.
Innovative Therapies for Bartonella
- Methylene Blue and Drug Combinations: Research from Johns Hopkins University has identified methylene blue as one of the most effective agents against stationary-phase Bartonella and its biofilm forms. Combinations such as azithromycin/methylene blue and rifampin/methylene blue have shown complete eradication of stationary-phase Bartonella in vitro.
These combinations offer a new line of defense, particularly in patients with relapsing or resistant infections.
- Biofilm Disruption: Recent studies are exploring biofilm disruptors like lumbrokinase and serrapeptase in combination with antibiotics to break down Bartonella biofilms, making the bacteria more susceptible to eradication.
Rickettsia: Expanding Beyond Doxycycline
While doxycycline remains the first-line treatment for Rickettsia infections like Rocky Mountain Spotted Fever, chronic forms and biofilm-related persistence may require alternative approaches.
New Research on Rickettsia Treatment
- Combination Therapies: Research suggests that combining rifampin or ciprofloxacin with traditional antibiotics may enhance the effectiveness of Rickettsia treatment, particularly in cases involving biofilm-related persistence.
- Adjunctive Therapies: Hyperthermia and IV therapies are being investigated as adjunctive treatments for severe or persistent Rickettsia infections.
Mycoplasma and Chlamydia: Addressing Intracellular Persistence
Mycoplasma and Chlamydia species are intracellular pathogens that complicate Lyme disease and its co-infections by evading the immune system and surviving inside host cells.
Advanced Therapies for Mycoplasma and Chlamydia
- New Antibiotics: Omadacycline and other next-generation tetracyclines have shown effectiveness against intracellular pathogens like Mycoplasma, offering improved outcomes in persistent infections.
- Immune Modulation: Immune-modulating therapies, such as low-dose naltrexone (LDN), are being used to boost the body's ability to fight chronic Chlamydia and Mycoplasma infections. These therapies, combined with antibiotics, may help clear long-standing infections that resist traditional treatment.
Anaplasma and Ehrlichia: Enhancing Immune Response
The treatment of Anaplasma and Ehrlichia relies heavily on doxycycline, yet immune support is increasingly recognized as a critical component in managing these infections.
New Treatment Considerations
- Cytokine Modulators: Cytokine modulation using advanced therapeutics, such as monoclonal antibodies or cytokine inhibitors, has shown promise in severe cases, especially where immune overactivation is driving pathology.
Biofilm Disruption: A Key to Treating Persistent Infections
Biofilm formation is a significant factor in the persistence of tick-borne pathogens like Borrelia, Bartonella, and Babesia. These protective structures shield bacteria from antibiotics and the immune system.
Modern Approaches to Biofilm Disruption
- Methylene Blue and Enzymatic Therapies: As shown in multiple studies, methylene blue can disrupt biofilm formation in Bartonella and Babesia, making it a key player in combination therapies.
- Enzyme-Based Therapies: Agents like lumbrokinase and serrapeptase are being used to break down biofilms, increasing the efficacy of antibiotics and helping clear chronic infections(
Immune Modulation and Integrative Approaches
Many chronic co-infections involve immune system dysfunction, where pathogens either evade detection or trigger excessive inflammation. Advanced therapies are now focusing on restoring immune balance.
Advanced Immune Therapies
- Low Dose Naltrexone (LDN): LDN is increasingly used to modulate the immune response in chronic Lyme and its co-infections. By reducing inflammation and supporting immune regulation, LDN helps the body fight infections more effectively.
- IVIG and Cytokine Therapy: In severe cases, intravenous immunoglobulin (IVIG) and cytokine therapies are being employed to restore immune function and mitigate the damage caused by chronic infections.
Experimental and Future Directions
Cutting-edge research is now exploring more experimental approaches for treating chronic tick-borne infections, particularly when traditional therapies fail.
Gene and Phage Therapy
- CRISPR and Gene Editing: Research into gene-editing technologies like CRISPR shows promise in targeting bacterial DNA directly, potentially eliminating pathogens at their genetic source.
- Phage Therapy: The use of bacteriophages—viruses that specifically target bacteria—is being explored as a future treatment for resistant bacterial infections like Borrelia and Bartonella.
Ivermectin as a Novel Treatment for Babesia Infections
Recent research has identified ivermectin (IVM) as a promising candidate in the treatment of Babesia species and Theileria, highlighting its broad antiparasitic effects beyond its traditional uses. In vitro studies demonstrated that IVM effectively inhibited the growth of Babesia species, including B. bovis, B. divergens, and B. caballi. In a mouse model infected with B. microti, IVM reduced parasitemia by 63%, and combination therapies with atovaquone (AQ) and diminazene aceturate (DA) further increased efficacy, reducing parasitemia by up to 83.7%. These findings suggest ivermectin could serve as an alternative or adjunct therapy for resistant Babesia infections.
Combination Therapies with Ivermectin
The study also explored combination therapies with ivermectin, such as IVM + DA and IVM + AQ, which proved particularly effective in reducing peak parasitemia in mice. These combinations not only diminished parasitemia but also resulted in the complete absence of B. microti DNA in treated groups after 49 days, indicating the potential for achieving a parasitological cure. This combination therapy offers a novel approach to treating Babesia infections resistant to traditional therapies.
Conclusion
The treatment of tick-borne co-infections requires a multi-faceted approach that integrates novel drug therapies, biofilm disruption, immune modulation, and emerging technologies. By leveraging cutting-edge research and clinical trials, we can move toward more effective, personalized treatments for patients with chronic Lyme disease and its associated co-infections.